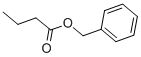
Benzyl Butyrate contains a molecular formula of C11H14O2, a boiling point of 240 °C, density of 1.009 g/mL, contains a boiling point between 238.00 to 240.00°C. A picture of benzyl butyrate is shown below:
Benzyl Butyrate is known to be a clear colorless liquid in appearance and contains a strong fruity odor like apricots. However, other researchers say that it smells like flowers. Benzyl butyrate is derived from benzyl alcohol and butanoic acid. The reaction for this is shown below:
CH3CH2CH2-COOH + C6H5CH2OH ----------------> C11H14O2
Benzyl Butyrate is soluble in alcohol and is used in perfume companion as a modifier for to benzyl acetate and benzyl propionate. In addition to this, the compound is used in flavoring as well as plactizier (something that makes plastic less rigid by breaking up the molecules). An example of this ester reacting to form a carboxylic acid derivative would be benzyl butyrate and benzyl alcohol can be converted to a carboxylic acid derivative by merely undergoing the reaction with a aldehyde which would give the carboxylic acid since aldehyde's convert benzene rings to carboxylic acids.
No comments:
Post a Comment